1.3 Elemental Composition of Pure Substances
7 min read•june 18, 2024
Dalia Savy
Jeremy Kiggundu
Dalia Savy
Jeremy Kiggundu
AP Chemistry 🧪
269 resourcesSee Units
Matter is any physical object that has mass and occupies space. It can be classified according to its state, its physical form, or its composition, the atoms or molecules that make it up.
Three States of Matter
The three states of matter are covered in greater depth in unit three, but here is a brief description of each:
🧊Solids
Solids are characterized by their ability to maintain a fixed shape and volume. This is because solids are composed of densely packed atoms held together by strong chemical bonds. Therefore, they only have slight movements known as vibrations.
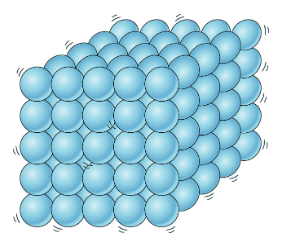
💧Liquids
Liquids are characterized by their ability to flow and take the shape of their container (fixed volume, no fixed shape). The particles in a liquid are less tightly packed together than in a solid, allowing them to move and flow past each other.
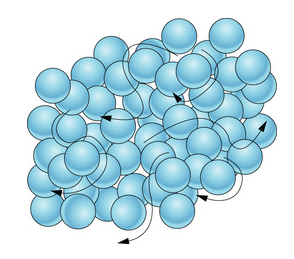
♨️Gases
Gases are characterized by their ability to expand and fill their closed container. The particles are very spread out and are in constant motion, moving in random directions and continuously colliding with each other and the walls of their container. Gases have no fixed volume or shape.
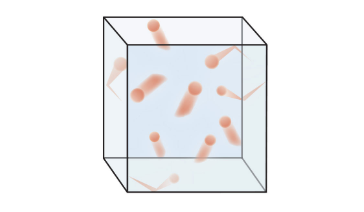
Categorizing Matter by Composition
The first division in the classification of matter by composition is between a pure substance and a mixture. Pure substances will be the focus of this section, while mixtures will be the focus of the next!
Pure Substances
Pure substances are substances that are composed of a single type of atom or molecule. Their composition does not vary from sample to sample.
Elements and Compounds
There are two types of pure substances: elements and compounds.
- Elements are composed of a singular type of atom, such as gold (Au), which is pure with regard to being composed of only gold atoms 🪙. Looking at the periodic table of elements, you can see every element that has been discovered by scientists.
- Compounds, on the other hand, are composed of atoms of two or more different elements bonded together.
- 💧 Water is an example of a compound and it is considered pure because water will always be made up of two hydrogen atoms and one oxygen atom, hence H2O.
- 🧂 Another example is table salt or sodium chloride. Every sample of NaCl is composed of one sodium ion bonded to one chloride atom.
Formula Units
Since compounds are made up of a unique ratio of different elements, there has to be a term to describe how they are held together in fixed proportions. A formula unit represents the lowest whole number ratio of atoms that can be used to describe the compound. In other words, it is the smallest collection of atoms from which the formula of a compound can be derived.
If we take a look at table salt again, the formula unit is 1:1. For every sodium ion, there is one chloride ion.
Formula units can allow us to take a look at the proportions of elements in a compound, rather than the individual particles themselves. Understanding them is useful as it can be used as another means of conversion.
Example Problem
How many formula units of KCl would be needed if 1.76 mol KCl were used in a chemical reaction?
Law of Definite Proportions
The law of definite proportions, or the law of constant composition, is one of the three fundamental laws that led to the development of the current atomic theory. It states that the ratio of the constituent elements in any pure chemical compound is always the same. Constituent elements are the elements that the compound is made up of. For example, no matter what sample of water you obtain, you will always have the same ratio of hydrogen to oxygen. Whether it be a 100 g sample, a 200 g sample, or a 5000 g sample, the ratio will always reduce to 2:1.
Understanding the Law of Definite Proportions 🍪
One way to understand the law of definite proportions is to compare it to a recipe. Just like a recipe species the exact proportions of every ingredient to make a particular meal or dessert, the law of definite proportions states that the ratio of elements in a pure compound is always going to be the same.
Think about a recipe for chocolate chip cookies. You'll never want to change the amount of flour, butter, eggs, and chocolate chips you use once you get the exact recipe right. You can make 100 cookies or 5 cookies, and the proportion of ingredients will always stay the same.
In the same way, the law of definite proportions states that the ratio of elements in a compound stays fixed. Otherwise, the compound would change in identity.
Empirical Formula
The empirical formula of a compound uses the very concept stated by the law of definite proportions. The empirical formula of a compound is the simplest whole-number ratio of the atoms of each element in a compound. In other words, it is a way to simplify the chemical formula of a compound.
The Empirical Formula of Glucose 🍬
Scientists often use the molecular formula of glucose (C6H12O6) rather than the empirical formula to properly represent the sugar on a molecular level. The empirical formula of glucose uses the factor of 2 to reduce the molecular formula down to the lowest whole-number ratio of each element: CH2O.
It is important to keep in mind that the empirical formula does not always reflect the actual number of atoms in a molecule, or even the arrangement of atoms in a molecule.
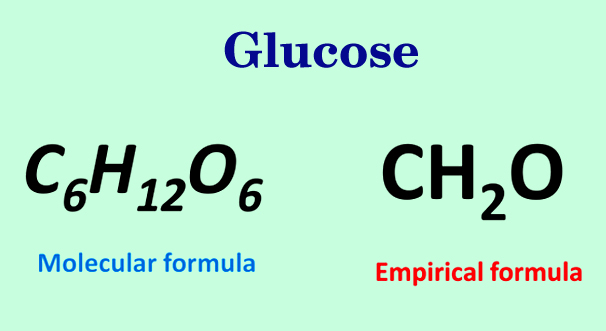
Image Courtesy of scienceabc
No matter how absurd the numbers in the molecular formula are, like C90H180O90, the ratio will always lead to the empirical formula of CH2O.
Empirical Formula - Practice Question #1
On the AP examination, you may be asked to derive the empirical formula based on a series of percent compositions given to you. Here is an example of this type of question:
(1) A carbohydrate, which contains C, H, and O, has a % composition of 33.3% C and 7.4% H. Find the empirical formula of this carbohydrate.
When taking a look at this type of question, try to remember the following "poem" as 4 steps:
Percent to grams, Grams to Moles, Divide by Small, Times to whole
Steps 1 and 4 are optional in this problem, but we are going to have to do both. Step 1 is only necessary if the composition is given in percentages. If it is already given in grams, you don't have to do it.
Step 1: Drop the %
To convert from percent to grams, simply drop the percent symbol! 33.3% C --> 33.3 g C and 7.4% H --> 7.4 g H.
But what about oxygen? We are told that the carbohydrate contains carbon, hydrogen, and oxygen, but we aren't given the percent composition of oxygen. There is one of the common errors that students make with these types of problems. Be sure to keep your eye out for each element and ensure you are accounting for all of them.
Let's use the concept that all elements in a compound must contribute to 100% of the mass of that said compound. In other words, all percentages have to add up to 100. Therefore, we can simply subtract 100%-33.3%-7.4% to obtain the percent composition of oxygen. This would get us 59.3% or 59.3 g O.
Step 2: Unit conversion
Now we have 33.3 g C, 7.4 g H, and 59.3 g O, and we have to convert all of these gram amounts to moles. This is done simply by dividing each of these gram amounts by the element's molar mass.
Remember, the molar mass of carbon is 12.01 g/mol, the molar mass of oxygen is 16.00 g/mol, and the molar mass of hydrogen is 1.008 g/mol.
C: 33.3 g / 12.01 g = 2.773 moles C
H: 7.4 g / 1.008 g = 7.34 moles H
O: 59.3 g / 16.00 g = 3.70625 moles O
Try not to round your answers until the very end to avoid using rounded numbers in your future calculations.
Step 3: Finding the lowest whole-number ratio
Now, we have to divide each mole amount by the smallest mole amount that we have. This allows us to reduce the chemical formula to the lowest whole-number ratio. There are fewer moles of carbon than there are of oxygen and hydrogen, therefore 2.773 is the smallest amount.
C: 2.773 / 2.773 = 1
H: 7.4 / 2.773 = 2.66
O: 3.70625 / 2.773 = 1.33
Step 4: Multiplying to a whole number
Since the ratios for hydrogen and oxygen aren't full numbers, we have to "times the numbers to whole numbers." Whenever the numbers end in 0.5, 0.33/0.66, or 0.25/0.75, multiply all by 2, 3, and 4, respectively. Here, since two of the values end in .33 or .66, we have to multiply all of them by 3 to get whole numbers.
C: 1 x 3 = 3 atoms C
H: 2.66 x 3 = 8 atoms H
O: 1.33 x 3 = 4 atoms O
Therefore, the empirical formula for this carbohydrate is C3H8O4. This question didn't ask for the molecular formula but an example would be C6H16O8. There are millions of possibilities for a molecular formula and they are all correct, as long as you multiply each subscript by the same number.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

© 2024 Fiveable Inc. All rights reserved.